Life at the Cell and Below-Cell Level. The Hidden History of a Fundamental Revolution in Biology
"Dr. Ling is one of the most inventive biochemist I have ever met." |
Chapter 6. Colloid, the Brain Child of a Chemist (p. 29-34) |
|
The discovery that cells are made of
protoplasm, a finding of critical importance to cell physiology, was promptly
seized upon and pursued with vigor and insight—not by a cell physiologist but
by Thomas Graham (1805-1869), a chemist. Graham was at the time Master of the
Mint of England,53 p 183
an office once held by Isaac Newton toward the end of the 17th century.464
p 229 6.1 Colloid, the name-sake of gelatin—and cogent model for
protoplasm Thomas Graham spent most of his life
investigating the phenomenon of diffusion. He noted that substances like
starch, gum and gelatin diffuse slowly and that they do not form crystals.
Graham wrote in 1861: "As gelatine appears to be
its type, it is proposed to designate substances of the class as colloids
(from Greek κδλλη: glue, or
gelatin, added by GL), and to speak of their peculiar form of
aggregation as the colloidal condition of matter."53 Martin Fischer, whose important contributions to cell
physiology will be reviewed below, defined colloids in these words:
"colloid systems result whenever one material is divided into a second
with a degree of division coarser than molecular."64 p 5 Ross Gortner, whose important work will also be presented below,
offered a modification: "colloidal systems result where one material is
divided into a second with a degree of subdivisions either (a) coarser than
molecular or (b) where the micelles exceed 1-1.5 millimicra
(10-15 Ǻ) in diameter." Gortner further
pointed out that an ultramicroscope makes visible
colloid particles from 10 Ǻ to 1000 Ǻ in diameter.64 p
5 Wolfgang Ostwald set the limits of
colloids between 10 Ǻ and 10,000 Ǻ.65 p 24
But another definition given by H. Staudinger
poses a special problem. By inventing colloid chemistry, Graham has
brought together two substances outstanding in the history of cellular and
subcellular physiology. They are copper ferrocyanide
and gelatin. We already know how copper-ferrocyanide
had launched the membrane theory. In the next section I shall review how
colloid chemists have discovered more and more intimate relationships between
gelatin and protoplasm. However, as will be made clear in [11.3(2)] following,
the time to construct a (plausible) theoretical explanation for this intimate
relationship was not to come until much later. 6.2. Coacervates (1) History In 1902, Pauli
and Rona added neutral salts to a solution of gelatin at 30°C and observed the
separation of the solution into two distinct layers. The bottom layer is rich
in gelatin; the top layer is gelatin-poor.58 In 1929 Bungenberg de Jong (1893-1977)
and H. Kruyt coined the term coacervation
for the phenomenon (from the Latin acervu
meaning aggregation and the prefix со, meaning together).59 Coacervate
is also used to designate the colloid-rich phase of the separated liquid. In
the case where salt-linkage formation between fixed anions and fixed
cations of the colloids plays a significant role in the colloid structure, the
coacervate is referred to as a complex coacervate. Colloid chemists in the past had called all proteins
colloids. With this in mind, one may think that all proteins can form
coacervates. This is not true. Only what Bungenberg
de Jong called linear proteins such as gelatin
form coacervates.61 p 185, p 239 Under conditions that promote the formation of coacervates
from linear proteins, most globular proteins form crystals instead.
This is a very important distinction to keep in mind. Most isolated native
proteins are globular. Gelatin is therefore unique or almost unique in maintaining
on a permanent basis a linear, or what I call fully-extended conformation
[11.2]. Why does gelatin assume and sustain such a fully-extended
conformation—a hitherto unanswered question—was given a possible explanation on the basis of new knowledge unknown until recently. And it will be
reviewed in [11.3(2)] to follow. (2)
Bungenberg de Jong's two
views on the physical state of water in coacervates Bungenberg de Jong offered
not one, but two theoretical interpretations for the structure of coacervates
and the physical state of water in the coacervate. In the old interpretation,
individual small colloidal particles with a diffuse solvate coating first join
together into larger particles with a clear boundary during the preparative
process. When these larger particles in turn join together to form coacervate,
their individual solvation shells merge to form an
overall shell with a concrete outer boundary (though no explanation was given
why a concrete boundary is formed). Note that in this (old) model, all or
nearly all of the water in a coacervate is not normal liquid water but hydration
water61 pp 245-246,
p 249 However, the new interpretation of coacervates, in Bungenberg de Jong's own words,
"stand(s) diametrically opposed to this (old) original idea" of water
in coacervates just described. Indeed, in the new model, "by far the
larger part (of water in the coacervate) is to be regarded as occlusion-water61 p 249
which is "Not bound to the macromolecules,"61 p 371
and therefore normal liquid water caught in between the network of
macromolecules. This new definition leaves one with the impression that there
is minimal interaction between the macromolecules and water in a
coacervate—quite the opposite of the old model. Fortunately, Bungenberg de Jong and his
coworkers have also left puzzled readers like myself
some quantitative data, which permits a deeper look into the subject. Holleman, Bungenberg de Jong and Modderman studied the
equilibrium distribution of sodium sulfate (Na2S04) in a
simple coacervate of gelatin + Na2S04 at 50°C.70
At a gelatin concentration of 27.2%, the concentration ratio of sodium sulfate
in the coacervate water and in external solution is 0.62. This partial
exclusion of Na2S04 indicates that of the 1 - 0.272 =
0.728 or 72.8% of water in the coacervate, 1 - 0.62 = 0.38 or 38% has no
solubility for sodium sulfate. Dividing the total amount of this water (equal
to 0.728 × 0.38 = 0.277) by the percentage of gelatin, one obtains
(0.277 / 0.272) = 1.02 grams of "non-solvent water for sodium sulfate"
per gram of dry gelatin. This figure is between 3 to 4 times larger than
the conventionally accepted (total) hydration water on native globular
proteins (i.e., 0.2 to 0.3 grams/gram of dry protein).155 Table
5 Nonetheless, there is also a difference between this realistic 38%
"hydration water" and the 100% "hydration water" as implied
in the old model. On the other hand, if the hydration water has higher than
zero solvency or q-value for Na2S04 {see
[11.3(4)]}, the departure from the old model could become smaller. We shall
return to this interesting subject in [11.3(3)] below.
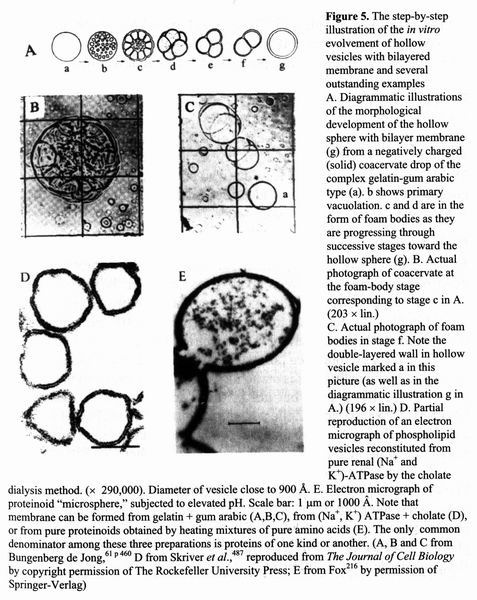
Figure 5. The step-by-step illustration of
the in vitro evolvement of hollow vesicles with bilayered membrane and
several outstanding examples A.
Diagrammatic illustrations of the morphological development of the hollow
sphere with bilayer membrane (g) from a negatively charged (solid) coacervate
drop of the complex gelatin-gum arabic type (a), b shows primary vacuolation. с and d are in the form of foam bodies as they are progressing through
successive stages toward the hollow sphere (g). B. Actual photograph of coacervate at the foam-body stage
corresponding to stage с in A. (203 × lin.) C. Actual
photograph of foam bodies in stage f. Note the double-layered wall in hollow
vesicle marked a in this picture (as well as in the diagrammatic illustration g
in A.) (196 × lin.) D. Partial
reproduction of an electron micrograph of phospholipid vesicles reconstituted
from pure renal (Na+ and K+-ATPase by the cholate dialysis method, (×
290,000). Diameter of vesicle close to 900 Ǻ. E. Electron micrograph of proteinoid
"microsphere," subjected to elevated pH. Scale bar: 1 um or 1000 Ǻ.
Note that membrane can be formed from gelatin + gum arabic (A,B,C), from (Na+,
K+) ATPase + cholate (D), or from pure proteinoids obtained by
heating mixtures of pure amino acids (E). The only common denominator among
these three preparations is proteins of one kind or another. (A, B and С from Bungenberg de Jong," 61 p 460
D from Skriver et al.,487 reproduced from The Journal of
Cell Biology by copyright permission of The Rockefeller University Press; E
from Fox216 by permission of Springer-Verlag) (3)
Coacervate and protoplasm If one mixes in the right proportion gelatin and gum arabic—a highly water-soluble, large complex polysaccharide from Acacia trees60 p 98—and allows the mixture to stand, two layers also separate out. If the test tube containing the layers is shaken, the gelatin-rich coacervate breaks up into many little balls or droplets61 p438 (see also Figure 5A), which stay undis-solved in the surrounding colloid-poor phase. In 1926 W.W. Lepeschkin
reported that the protoplasm oozing out from broken (young) cells of the plant,
Bryopsis plumosa
can also be shaken and broken up into many little balls.62 p
75 They too stay undissolved in the surrounding
aqueous medium. As judged by these strikingly similar characteristics, the
gelatinous materials emerging from crushed protozoa by Felix Dujardin and Willy Kiihne and
from broken plant cells by von Nageli, von Mohl, Lepeschkin as well as
Kuroda (who produced Figure 3) must all be coacervates. This is not a new idea.
Lepeschkin was among the firsts to suggest that
protoplasm is a coacervate.324 (4)
Coacervate and the living cell In a review written for the journal, Protoplasma,71
Bungenberg de Jong cited
nine similarities between coacervates and what he called a static model of
the living cell, including (i) water
immiscibility, (ii) tendency to form vacuoles, (iii) tendency to engulf solid
particles, (iv) behaviors under the influence of a direct-current (DC) electric
field. Bungenberg de Jong then pointed out that the most basic difference between the living cells and his static model lies in the possession of membranes in the living cells but not in the coacervates ("Der wesentliche Unterschied der lebenden Zeile gegemiber unserem statischen Modell bezieht sich wohl aufdas Vorhandensein von Filmen oder Membranene in ersteren, die grundsatzlich Ungleichgewicht ermoglichen.").71 p 164 Why Bungenberg de Jong made this distinction is a mystery because he himself has shown how, under the right conditions, coacervate can form membranes too (Figure 5A and 5C and legend). In a following section I shall present the work and ideas of A. S. Troshin, who saw perhaps even greater significance in Bungenberg de Jong's work on coacervates than Bungenberg de Jong did himself. |
|
|
Разделы книги
Contents (PDF
218 Kb) |
|
|
На страницу
книги "Life at the Cell and Below-Cell Level..." |
|