Life at the Cell and Below-Cell Level. The Hidden History of a Fundamental Revolution in Biology
"Dr. Ling is one of the most inventive biochemist I have ever met." |
Chapter 15. Physiological Activities: Electronic Mechanisms and Their Control by ATP, Drugs, Hormones and Other Cardinal Adsorbents (p. 179-232) |
|
The membrane theory once gained world-wide
acceptance for its success in explaining four major physiological
manifestations of the living cells: solute distribution, permeability, swelling
and shrinkage and resting potential. The demonstration that the cell membrane
is in fact permeable to sucrose and Na+ made the original membrane
theory difficult to defend. In response, the sodium pump hypothesis was
installed. In time, this subsidiary hypothesis also met serious
contradictions, even harder to defend. And with it, the paradigm of cells as
membrane-enclosed dilute solutions is, in my opinion, coming to an end. In presenting the LFCH and the PM theory, we focused
primarily on two basic cell physiological phenomena: selective K+
adsorption and Na+ exclusion on one hand; multilayer polarization
and orientation of cell water on the other. Both are static
manifestations of the resting living state. The first part of the present
chapter offers new interpretations of the four classic static physiological
manifestations mentioned above: solute distribution, permeability, swelling and
shrinkage and resting potential in the light of the AI Hypothesis. We then
examine how these physiological manifestations are controlled by ATP, drugs,
hormones or other cardinal adsorbents through the operation of the electronic
control mechanism described in the preceding chapter. In addition, I shall also
tackle two dynamic cell physiological activities: the action
potential and true active transport.
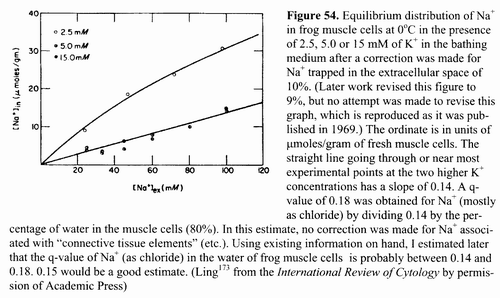
Figure 54. Equilibrium distribution of Na+ in frog
muscle cells at 0°C in the presence of 2.5, 5.0 or 15 mM
of К+. in the bathing medium after
a correction was made for Na+ trapped in the extracellular space of
10%. (Later work revised this figure to 9%, but no attempt
was made to revise this graph, which is reproduced as it was published in
1969.) The ordinate is in units of μmoles/gram
of fresh muscle cells. The straight line going through or near most
experimental points at the two higher K+ concentrations has a slope
of 0.14. A q-value of 0.18 was obtained for Na+ (mostly as chloride)
by dividing 0.14 by the percentage of water in the muscle cells (80%). In this
estimate, no correction was made for Na+ associated with
"connective tissue elements" (etc.). Using existing information on
hand, 1 estimated later that the q-value of Na+ (as chloride) in the
water of frog muscle cells is probably between 0.14 and 0.18. 0.15 would be a
good estimate. (Ling173 from the International Review of Cytology
by permission of Academic Press) 15.1
Selective solute distribution in living cells; cooperativity
and control In this section, we resume our discourse on
the distribution of Na+ and K+ (and Mg2+) in
frog muscle cells. More specifically, we focus attention on the influence of ATP
on the state of cell water and K+; and the influence of the cardiac
glycoside, ouabain, on the accumulation of this and other alkali-metal ions in
frog muscles. After that, I shall discuss the accumulation in frog
muscle cells of D-glucose and the free amino acid, glycine; and their
respective control by the hormone, insulin. In addition, I shall also touch
upon lactose accumulation in the bacteria, Escherichia coli (E. coli). (1)
K+, Na+ and Mg2+ accumulation in living cells In a preceding section [10.2], I have
devoted much space establishing the adsorbed state of cell K+. We
now turn our attention to Na+ and Mg2+ which so far have
been kept on a back burner. Figure 54 shows the equilibrium distribution of Na+
in frog muscle at 0°C in the presence of a normal external K+ concentration
(2.5 mM) and higher ones.173 The data show
general obedience to the Troshin equation (Equation
Al in Appendix 1), which describes a free fraction of Na+ in the
cell water and another fraction of adsorbed Na+. After the adsorbed fraction of Na+ is chased away by K+ at the higher concentrations, a straight-line plot of the remaining Na+ in the tissue water against external Na+ concentration is obtained with a slope of 0.14. After corrections for Na+ trapped in the extracellular space and adsorbed on "connective tissue elements," one obtains a q-value of 0.15 for Na+ (as chloride) in frog muscle cell water. The q-value for sucrose in frog muscle cell water at the same temperature (0°C) is, as seen in Figure 27, 0.13.156 p 191 While the distribution of (univalent) Na+
in frog muscle depends strongly upon the concentration of (univalent) K+, the
distribution of (divalent) Mg2+ in both frog muscle and frog
ovarian eggs is indifferent to the concentration of (univalent) K+
as the studies of Ling, Walton and Ling have revealed.502 Similarly,
the distribution of K+ is also indifferent to the concentration of Mg2+
in the bathing medium. This mutual indifference is in conflict with the
membrane theory as expounded by Donnan [4.3] where
the same Donnan ratio governs the distribution of all
permeant ions 98 p 216; 15 p 28; 107 p 17
as well as the resting potential (Figure
4B). By the same token, it is in harmony with the AI Hypothesis, according to
which there is no causal relationship between the resting
potential and bulk phase ionic distribution, nor obligatory
relationship between monovalent and divalent ion distribution as they adsorb in
the cells on separate and different types of sites. In frog muscle there are about 12 mmoles of Mg2+
adsorbing sites with strong affinity for this ion—reaching full saturation at
an external Mg2+ of 1 mM (or less) (1 mM is the lowest concentration we studied). The q-value of Mg2+
(as chloride) is 0.21 at 25°C. To a first approximation, the data can also be
described by the Troshin equation. The
Troshin equation is a special case of Ling's general
equation for solute distribution in living cells and model systems156
(presented as Equation A3 in Appendix 1). In the theory, which the Troshin equation stands for, there is no cooperative
interaction among the adsorption sites. In the Al Hypothesis, on the other
hand, cooperativity among the β- and
γ-carboxyl and other proximal
functional groups is a universal feature. But it may be undetectable
under some conditions. Autocooperativity becomes detectable when the nearest
neighbor interaction energy is much larger than zero (-γ/2 >> 0).
-γ/2 is much larger than zero, when the alternative adsorbents have widely
different adsorption energies (for details, see Reference 107 p 139-140), as in
the case when frog muscles are in an environment containing a high
concentration of Na+ and very low K+ concentration.
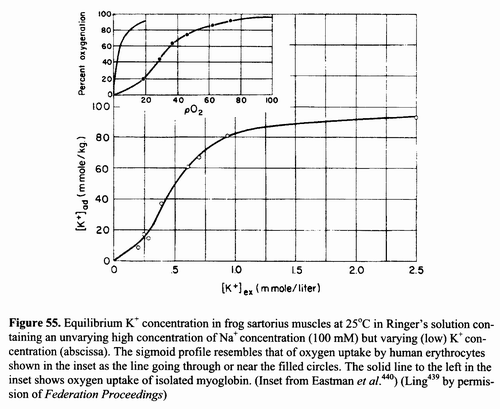
Autocooperativity is evident in the (sigmoid) uptake
curve of K+ in Figure 55, which resembles the oxygen uptake curve of
human erythrocytes shown in the inset. In contrast, oxygen uptake of myoglobin—shown on the left of the inset—does not exhibit
autocooperativity. Instead, it follows a
"hyperbolic" uptake curve characteristic of Langmuir
adsorption (with no discernible near-neighbor interaction).
To be continued |
|
|
Разделы книги
Contents (PDF
218 Kb) |
|
|
На страницу
книги "Life at the Cell and Below-Cell Level..." |
|